|
|
Chemdea |
|
PX-866 |
Catalog # CD0279 |
|
Price: Inquire |
Availability: Inquire |
|
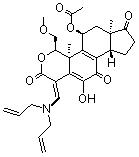
(1E,4S,4aR,5R,6aS,9aR)- 5-(Acetyloxy)-1-[(di-2-propen-1-ylamino)methylene]-4,4a,5,6,6a,8,9,9a-octahydro-11-hydroxy-4-(methoxymethyl)-4a,6a-dimethylcyclopenta[5,6]naphtho[1,2-c]pyran-2,7,10(1H)-trione Yellow solid. PROTECT FROM LIGHT. PACKAGED UNDER INERT GAS. Purity: 98% by HPLC. CAS# 502632-66-8. Solubility: DMSO, MeOH. Molecular Formula: C29H35NO8. Molecular Weight: 525.6.
Biologically
stable, semisynthetic derivative of wortmannin. PX-866 is more potent that
its parent compound, wortmannin. PX-866 is a PI3-K inhibitor with selectivity
for p110α. Administered to
mice at 10 mg/kg inhibited phospho-Ser473-Akt in HT-29 colon tumor xenografts
up to 80% with recovery taking >48 hours after p.o. administration but
more rapidly after i.v. or i.p. administration. PX-866 exhibited in vivo
antitumor activity against A major toxicity of PX-866 administration
was hyperglycemia with decreased glucose tolerance, which was reversed upon
cessation of treatment. The decreased glucose tolerance caused by PX-866 was
insensitive to the AMP-activated protein kinase inhibitor metformin but
reversed by insulin and by the peroxisome proliferator-activated receptor-γ
activator pioglitazone. PX-866 increases
the antitumor effects of cisplatin and radiation treatment. References: 1. Ihle, N. T. et al Mol. Canc. Ther. 2009, 8, 94; 2. Howes, A. L. et al Mol. Canc. Ther. 2007, 6, 2505; 3. Ihle, N. T. et al Mol. Canc. Ther. 2005, 4, 1349; 4. Ihle, N. T. et al Mol. Canc. Ther. 2004, 3, 763. |
|
SAME PRODUCT AS: |
||
|
Cat. No.
N/A |
Cat. No. N/A |
Cat. No. N/A |
|
Cat. No.
N/A |
Cat. No.
N/A |
Cat. No.
N/A |
|
CHEMDEA; |